biuret reagent contains|What does Biuret test for? Its Principle, Mechanism and Uses : Baguio Biuret Reagent. The Biuret reagent is a solution composed of sodium hydroxide (NaOH) or potassium hydroxide (KOH), hydrated copper (II) sulfate, and potassium sodium tartrate. Bokep Sedarah Viral - Situs Nonton Video Bokep Asian,Streaming Vidio Bokep Asia Terbaru,Download Bokep Asia Tante,Bokep Memek Asia Indo,Bokep Indonesia Viral Kualitas Full HD Gratis . Bokep sedarah indonesia puasin kakak ipar. 698K 100%. HD 01:51. bokepskandal selingkuh bibi dan keponakan. 540K 100%. HD 02:00. Bokep .
PH0 · What does Biuret test for? Its Principle, Mechanism and Uses
PH1 · What does Biuret test for? Its Principle, Mechanism and
PH2 · Biuret test
PH3 · Biuret Test: Principle, Reagent, Procedure &Result Interpretation
PH4 · Biuret Test: Principle, Reagent, Procedure &Result
PH5 · Biuret Test: Principle, Procedure, and Uses • Microbe Online
PH6 · Biuret Test: Principle, Procedure, and Uses •
PH7 · Biuret Test for Protein: Principle, Procedure, Results, Uses
PH8 · Biuret Test for Protein
PH9 · Biuret Test
PH10 · 2.5: Proteins
PH11 · 1.3.5 Biochemical Tests: Proteins
POP! Slots Free Chips - myVEGASadvisor POP! Slots Free Chips is your ultimate source of free chips for the popular mobile slot game. You can collect free chips daily, play exciting slots, and redeem amazing rewards such as Las Vegas buffets, hotels and more. Don't miss this chance to experience the thrill of Vegas on your phone.
biuret reagent contains*******The reaction in the biuret test is a colorimetric reaction where the result is indicated by a color change from blue to purple or violet. In an alkaline environment, the cupric (Cu+2) ions in the biuret reagent bind to the nitrogen atoms in the . Tingnan ang higit paThe Biuret reagent is made of sodium hydroxide (NaOH) and hydrated copper(II) sulfate, together with potassium sodium tartrate, the latter of which is added to chelate and thus stabilize the cupric ions. The reaction of the cupric ions with the nitrogen atoms involved in peptide bonds leads to the displacement of the peptide hydrogen atoms under the alkaline conditions. A tri- or tetra-dentate chelation with the peptide nitrogen produces the characteristic color. This is found with dipeptides.biuret reagent contains What does Biuret test for? Its Principle, Mechanism and UsesTest for protein. A liquid solution of a sample is treated with sodium or potassium hydroxide to make the solution alkaline. A few drops of copper (II) sulfate solution (which is blue) is . Biuret Reagent. The Biuret reagent is a solution composed of sodium hydroxide (NaOH) or potassium hydroxide (KOH), hydrated copper (II) sulfate, and potassium sodium tartrate.
The Biuret Test for Protein is based on the principle that copper ions in the Biuret reagent react with peptide bonds to form a violet-coloured complex. The Biuret reagent contains sodium hydroxide, .
The biuret test is a colorimetric test that helps detect specific proteins or peptide bonds in given analytes. It is followed by spectrophotometry for quantification. The test requires the use of a biuret reagent. This . Biuret reagent contains what ions? Biuret reagent contains copper ions (Cu 2+ ) which react with peptide bonds present in proteins to form a complex that absorbs light at a wavelength of around 540 .
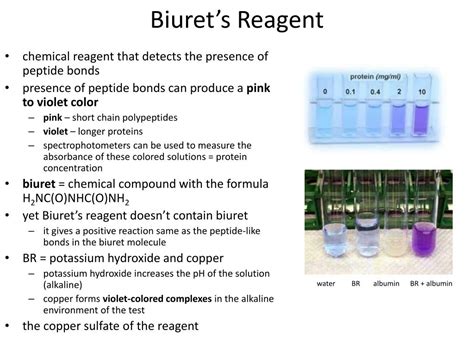
Biuret test uses a reagent consists of potassium hydroxide and copper sulfate. Under normal condition, the color of the biuret reagent is blue.
Biuret test is used for the detection of peptide bonds in the protein. This test is not suitable for amino acid only. The protein solution when reacts with CuSO4 in alkali condition .
These peptide bonds are unique to proteins and can be detected using Biuret reagent. Biuret reagent is normally a pale blue color but will turn into a light pink color in the .What does Biuret test for? Its Principle, Mechanism and Uses Overview of Biuret Test. Biuret is a compound produced by heating urea at 180 ℃. Biuret test is the name of a chemical test that utilises the Biuret reagents, which contains a 1% solution of Copper II sulphate (CuSO₄). It is the Cu₂⁺ in the Biuret reagent that forms a complex with the peptide bonds found in proteins. Hence, this test .A positive reaction for this test is also received when the analyte contains biuret molecules ([H 2 N-CO] 2 NH) since the bonds in this molecule are similar to peptide bonds. Biuret Reagent. The biuret reagent is made .
The biuret test is a chemical test for proteins and polypeptides. It is based on the biuret reagent, a blue solution that turns violet upon contact with proteins, or any substance with peptide bonds. The test and reagent do not actually contain biuret; they are so named because both biuret and proteins have the same response to the test. History Biuret reagent is an alkaline solution of 1% CuSO 4, copper sulfate. The violet color is a positive test for the presence of protein, and the intensity of the color is proportional to the number of peptide bonds in the solution. Biuret Test. Examine the table below. Indicate if the sample is a negative control, positive control or an experimental.
biuret reagent contains Principle of Biuret test: Biuret test is a general test for compounds having a peptide bond. Biuret is a compound formed by heating urea to 180° C. When biuret is treated with dilute copper sulfate in alkaline condition, a purple colored compound is formed. This is the basis of biuret test widely used for identification of proteins and amino .
Reagents: Sodium hydroxide, copper sulfate, . The RIPA buffer contains ionic (sodium dodecyl sulfate, SDS) as well as nonionic (Triton X-100) detergents that disrupt the membrane and separate the membrane-bound, cytoplasmic proteins in the buffer. . In the biuret test, the sample is treated with an alkaline copper sulfate reagent that .
The biuret reagent contains copper ions, or charged atoms of copper. The charge on the ions of copper are +2. When the copper ions come in contact with peptide bonds, the charge changes to +1 and .
Biuret Reagent 110 ml (Product Code B 3934) Reagent contains 0.75 mM cupric sulfate and 94 mM sodium hydroxide. It also contains tartrate, iodide, and carbonate. Folin and Ciocalteu's Phenol Reagent 5 ml (Product Code F 6678) 2.0 Normal Protein Standard 5 ml (Product Code P 9369) The standard contains 100 mg/ml of bovine serum
The last episode of “The Marilyn Denis Show,” featuring Eugene Levy and Andrea Martin, aired on June 9.
biuret reagent contains|What does Biuret test for? Its Principle, Mechanism and Uses